The Phenomenon of Memory
Think back on a happy memory. While you may not be able to recall the details vividly, you will probably be able to remember what made you happy, as well as a general recollection of the event’s context. Now try to think back to what you ate for lunch two weeks ago—odds are you will not remember. The concept of long-term memory formation is an incredible phenomena. It is the biological process of storing and retrieving information that, although seemingly trivial and second-nature, functions as the foundation for shaping one’s identity and informing one’s decision-making. However, the mechanisms and neurobiology behind this remarkable ability remain far from understood. How is it possible for the assembly of atoms and molecules that comprises the human brain to have the capacity to “remember?” Novel research in this branch of neuroscience may provide a glimpse into exactly how a collection of neurons across the brain’s neural network work in tandem to make this process possible.
How do Memories Form?
While scientific research on long-term memory formation has been underway for decades, the underlying mechanisms by which this process occurs have yet to be definitively proven. However, an abundance of cutting-edge research supports the current dominating theory on long-term memory formation, which revolves around a revolutionary concept termed “synaptic plasticity.”
Synaptic plasticity refers to the ability for neurons to strengthen or weaken their connections with one another in response to an increase or decrease in their activity. Essentially, if neuron 1 consistently succeeds in activating neuron 2, the connection between the two becomes stronger. Likewise, if neuron 1 fails in activating neuron 2, the connection becomes weaker. As these connections are developed, memories become encoded within the brain’s neural network. These lasting increases or decreases in synaptic strength are called “long-term potentiation” (LTP) and “long-term depression” (LTD) respectively and are influenced by a variety of neurobiological factors.1
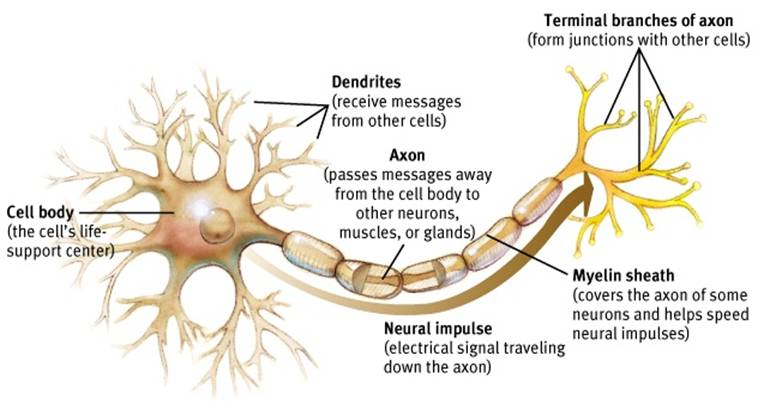
One such factor recently discovered to play an important role in long-term memory formation is the neuronal PAS domain protein 4 (Npas4). Within the hippocampal region of the brain—the region primarily responsible for learning, memory formation, and storage—Npas4 was found to be directly responsible for synaptic maintenance. Npas4 plays an important role in memory formation through its regulation of plk2, a gene responsible for controlling the shrinking of the postsynaptic structures of neurons. As Npas4 activates plk2, synaptic size and strength decreases. This suggests that without Npas4, synapses would become excessively strong, inhibiting their capacity to encode memories through further strengthening.2 Due to its remarkable ability to control the strength of connections between neurons, Npas4 plays an integral role in synaptic plasticity and the complex process of long-term memory formation.
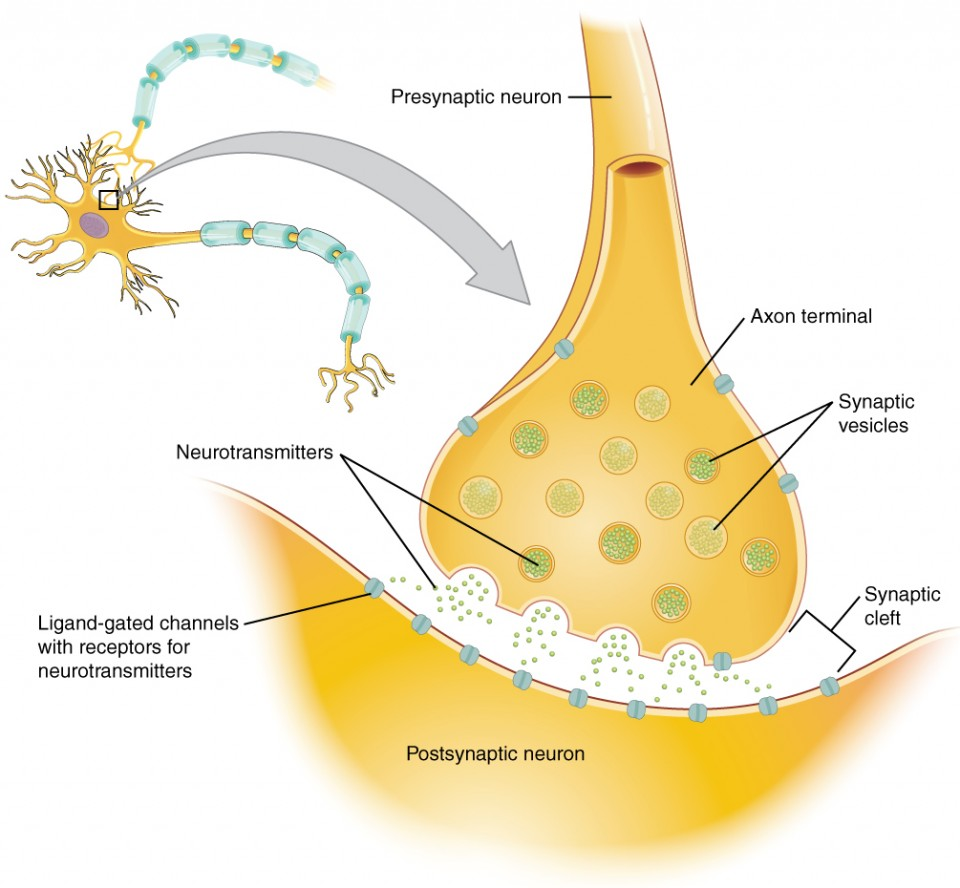
An additional neurobiological factor with important ties to memory formation is the protein Staufen2. While studying this protein, researchers were able to pinpoint its impact on the efficiency of signal transmission across synapses in the hippocampus. It was found that synthetically reduced levels of Staufen2 within mice enhanced LTP and impaired LTD, which had a negative impact on memory. This implies that the deficiency of Staufen2 makes synapses more responsive than normal. Researchers hypothesize that as synapses become highly responsive, not enough become suppressed during the process of memory consolidation in which experiences are encoded into long-term memory. These findings illustrate that the absence of Staufen2 and the consequent imbalance in LTP and LTD may lead to the destabilization of long-term memory formation.3
Although Staufen2 and Npas4 are both examples of proteins that play a pivotal role in the process of memory formation, it is important to note they are but a small part of the molecular machinery involved with memory. The complex process of long-term memory formation relies on an incredible array of intricate interactions between neurobiological processes that work in tandem to strengthen connections across the neural network and encode a person’s experiences into long-term memories. Understanding how synaptic plasticity works may provide the foundation for not only understanding human memory but also the neurodegenerative disorders that can result from a fault in these complex processes.
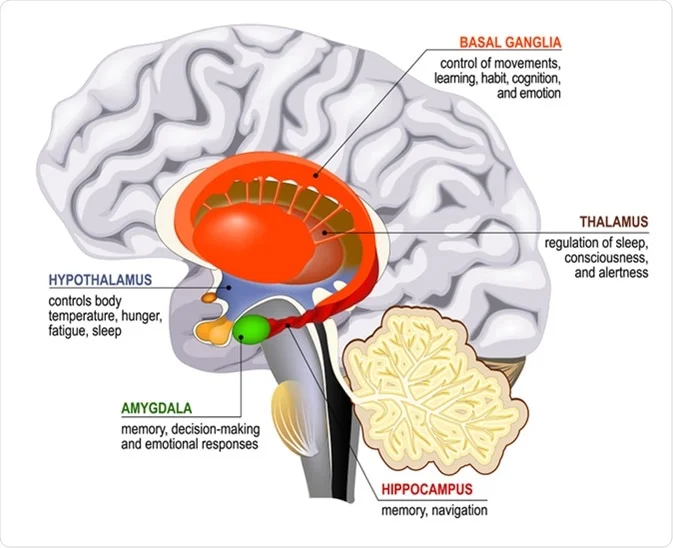
Where can Memory Formation and Retention go Wrong?
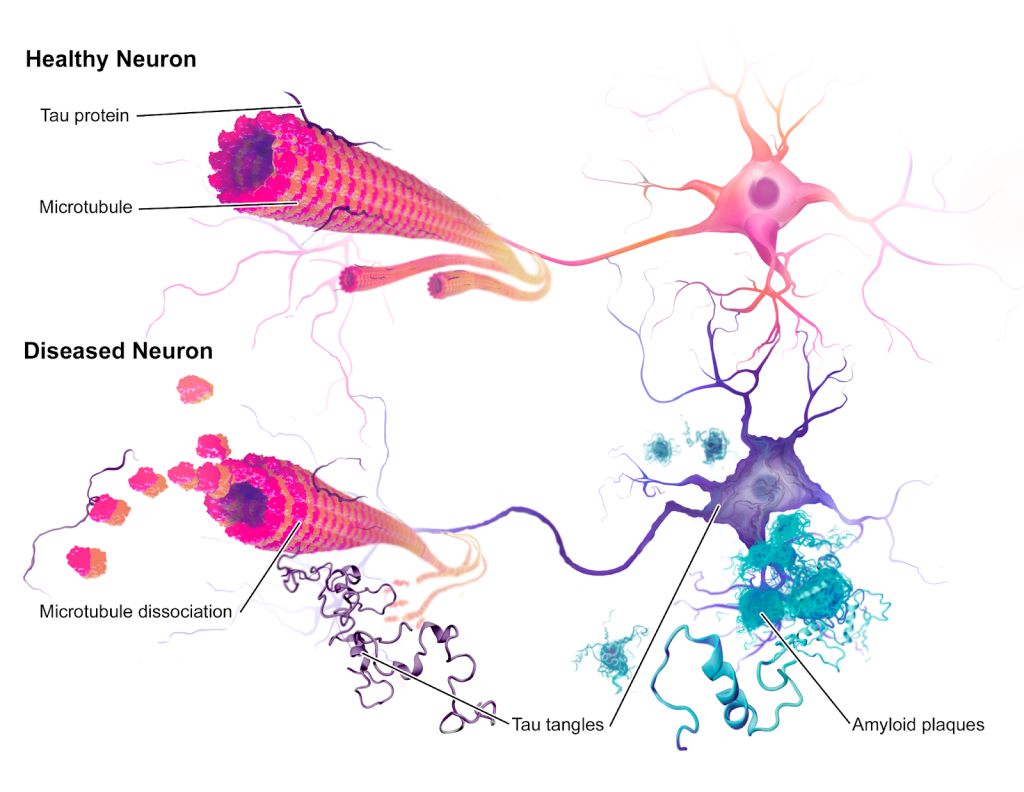
Alzheimer’s disease (AD) is one of the most common forms of a neural network malfunction in which brain cells degenerate and die. This neurodegenerative disease may cause symptoms such as severe memory loss, the inability to form new memories, and erratic changes in behavior. Moreover, Alzheimer’s disease is the cause of 60%-80% of all dementia cases and typically leads to a fatal prognosis after 10 years of onset.4 The hallmarks and neurobiology of AD are centered around the development of two toxic proteins: amyloid-beta proteins and tau proteins. These result in the problematic growth of amyloid-beta plaques and neurofibrillary tangles respectively. Although it is not clear to what extent each of these pathologies—plaques or tangles—causes AD, both are suspected to contribute to the deterioration of cognitive functions, including the ability to form and recall memories.
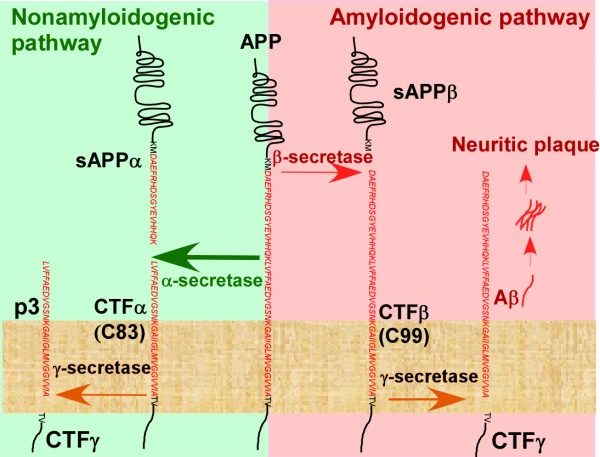
In amyloid-beta plaque formation, the gradual buildup of plaque from small molecules of amyloid-beta (Aβ) proteins covers large portions of the brain. This serves as a marker for the progression of AD and possibly leads to neural cell death as well. Aβ proteins are formed when a large protein involved in neural growth and neurorepair, amyloid-beta precursor protein (APP), is broken down via two distinct pathways.5
In the first pathway—generally regarded as “non-amyloidogenic” for its hypothesized non-toxic products—APP is cleaved in two by the enzyme ɑ-secretase. This results in the formation of the protein fragments C83 and sAPPɑ, the latter of which is hypothesized to have neuroprotective effects. C83 is again cleaved by γ-secretase, resulting in a new protein called p3. Although largely regarded as benign, research is starting to shed light on the specific effects of the accumulation of p3, as it may actually have toxic, amyloidogenic properties. Previous studies have found that this protein may be linked to the formation of aggregates and amyloid fibrils, and thus may not be non-amyloidogenic as previously thought.6
In the second pathway—which may be associated with genetic abnormalities—APP is instead cleaved by a different enzyme, β-secretase, forming sAPPβ and a protein fragment called C99.7 Next, C99 is cleaved by γ-secretase, resulting in another protein fragment and the 42-amino acid Aβ peptide. As the function of a protein is determined by its specific amino acid sequence and how these amino acids organize, Aβ is considered toxic due to its resulting insolubility and its tendency to form long fibrils that create dense plaques on neural cells, which interfere with synaptic function.8 This gradual accumulation of plaque in the brain may result in decreased cognitive function and ultimately lead to neural cell death.9
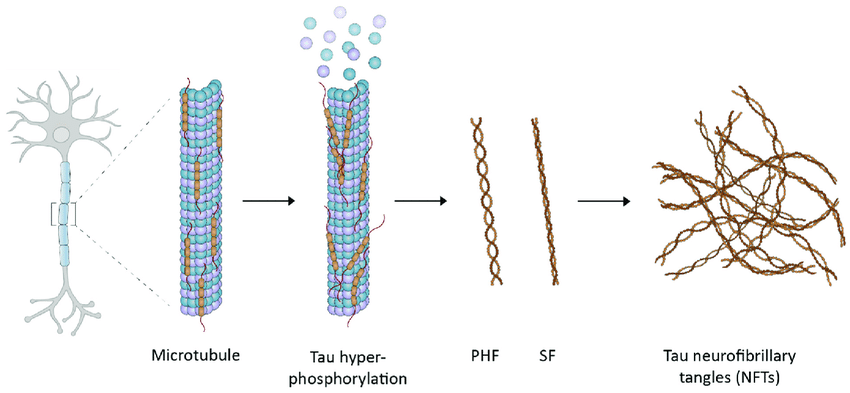
An additional characteristic of Alzheimer’s disease is the presence of neurofibrillary tangles (NFTs), which are the result of the aggregation of tau proteins within neurons. The normal function of tau proteins is to form the cytoskeleton, or the framework, of a neuron. Tau proteins carry out this function by promoting the development and stabilization of microtubules—structures which provide support for a neuron and facilitate transportation of substances, such as neurotransmitters, across the cell. However, as tau proteins become impaired during the progression of AD, its function changes and its capacity to bind to and support microtubules becomes hindered. As it falls off the microtubule, it then begins to aggregate, forming a series of tangles which negatively affect neuronal function.10
Although the neurobiological mechanisms facilitating the progression of tau pathology are still unclear, evidence suggests the presence of abnormal tau proteins with extra phosphate groups attached (termed hyperphosphorylated) may be a contributing factor.11 In fact, following studies by Ksiezak-Reding et al., 6-8 mol of phosphate per molecule of tau were found in the autopsied brain of an individual suffering from Alzheimer’s, as opposed to 2-3 mol in a healthy brain.12 Because tau phosphorylation is regulated by the activities of cellular enzymes and protein kinases—the molecules which facilitate the attachment of phosphate groups—an imbalance could potentially result in the neurotoxic hyperphosphorylation of tau proteins, and the subsequent impairment of cognitive function.13
Conclusion
The mechanism by which memories are encoded via the synaptic plasticity of the brain’s neural network is an incredible process—it brings to light various questions revolving around the molecular machinery which makes this function of memory possible. Moreover, by studying the intertwining nature of synaptic plasticity and neurodegenerative diseases, promising possibilities exist in developing therapies for common pathologies such as Alzheimer’s disease. Although no cure has been developed for AD, a treatment does exist that helps slow its progression. The Aducanumab medication, the only currently approved treatment, is a form of immunotherapy which targets the Aβ protein to reduce the formation of amyloid plaques in the brain.14
Although it may seem diminutive to reduce one’s most defining memories to mere electrical impulses and interactions of proteins, it is fascinating to realize that these neurobiological phenomena serve as the foundational mechanisms which govern one’s identity and thinking. Uncovering the complexities of what allows us to remember is an essential stepping stone; it provides the key towards unlocking not only a greater hope for effectively treating those with neurodegenerative diseases, but also towards providing a greater understanding of human thinking and the basis of what makes us who we are.
Acknowledgements
I would like to thank and acknowledge Dr. William Jagust, professor of public health and neuroscience at UC Berkeley, for his helpful feedback and expertise in Alzheimer’s research, which helped greatly in the writing process. I’d also like to thank my editors Jonny Hale and Marley Ottman, of the Berkeley Scientific Journal Features Department, for their thorough edits and guidance throughout the writing process.
References
- Queensland Brain Institute. (2016, December 2). How are memories formed? The Brain. https://qbi.uq.edu.au/brain-basics/memory/how-are-memories-formed
- Trafton, A. (2018, February 8). Study reveals molecular mechanisms of memory formation. MIT News. https://news.mit.edu/2018/study-reveals-molecular-mechanisms-memory-formation-0208
- Ludwig-Maximilians-Universitaet Muenchen. (n.d.). Neurobiology: The chemistry of memory. ScienceDaily. Retrieved March 14, 2022, from https://www.sciencedaily.com/releases/2017/11/171123095409.htm
- American Brain Foundation. (n.d.). Alzheimer’s disease. Brain Diseases. Retrieved March 14, 2022, from https://www.americanbrainfoundation.org/diseases/alzheimers-disease/
- Dawkins, E., & Small, D. H. (2014). Insights into the physiological function of the β-amyloid precursor protein: Beyond Alzheimer’s disease. Journal of Neurochemistry, 129(5), 756–769. https://doi.org/10.1111/jnc.12675
- Kuhn, A. J., Abrams, B. S., Knowlton, S., & Raskatov, J. A. (2020). The Alzheimer’s disease “non-amyloidogenic” p3 peptide revisited: A case for amyloid-α. ACS Chemical Neuroscience, 11(11), 1539–1544. https://doi.org/10.1021/acschemneuro.0c00160
- Robertson, S. (2014, January 17). What are amyloid plaques? News Medical Life Sciences. https://www.news-medical.net/health/What-are-Amyloid-Plaques.aspx
- Goodsell, D. (2006, July). Molecule of the month: Amyloid-beta precursor protein. RCSB PDB-101. http://pdb101.rcsb.org/motm/79
- Zhang, X., & Song, W. (2013). The role of APP and BACE1 trafficking in APP processing and amyloid-β generation. Alzheimer’s Research & Therapy, 5(5), 46. https://doi.org/10.1186/alzrt211
- Iqbal, K., Liu, F., Gong, C.-X., & Grundke-Iqbal, I. (2010). Tau in Alzheimer disease and related tauopathies. Current Alzheimer Research, 7(8), 656–664. https://doi.org/10.2174%2F156720510793611592
- Alonso, A. D., Cohen, L. S., Corbo, C., Morozova, V., ElIdrissi, A., Phillips, G., & Kleiman, F. E. (2018). Hyperphosphorylation of tau associates with changes in its function beyond microtubule stability. Frontiers in Cellular Neuroscience, 12. https://www.frontiersin.org/article/10.3389/fncel.2018.00338
- Chong, F. P., Ng, K. Y., Koh, R. Y., & Chye, S. M. (2018). Tau proteins and tauopathies in Alzheimer’s disease. Cellular and Molecular Neurobiology, 38(5), 965–980. https://doi.org/10.1007/s10571-017-0574-1
- Wang, J.-Z., Grundke-Iqbal, I., & Iqbal, K. (2007). Kinases and phosphatases and tau sites involved in Alzheimer neurofibrillary degeneration. European Journal of Neuroscience, 25(1), 59–68. https://doi.org/10.1111/j.1460-9568.2006.05226.x
- National Institute on Aging. (n.d.). How is Alzheimer’s disease treated? Health Information. Retrieved May 19, 2022, from https://www.nia.nih.gov/health/how-alzheimers-disease-treated
Image References
Figure 1: AP Psychology Community. (2022, March 16). https://appsychology.com/
Figure 2: Lumen. (n.d.). Anatomy and physiology – communication between neurons. https://courses.lumenlearning.com/ap1/chapter/communication-between-neurons/
Figure 3: Sinha Dutta, S. (2010, May 4). Hippocampus functions. News Medical Life Sciences. https://www.news-medical.net/health/Hippocampus-Functions.aspx
Figure 4: Zhang, X., & Song, W. (2013). The role of APP and BACE1 trafficking in APP processing and amyloid-β generation. Alzheimer’s Research & Therapy, 5(5), 46. https://doi.org/10.1186/alzrt211
Figure 5: Jie, C., Treyer, V., Schibli, R., & Mu, L. (2021). TauvidTM: The first FDA-approved PET tracer for imaging tau pathology in Alzheimer’s disease. Pharmaceuticals, 14, 110. https://doi.org/10.3390/ph14020110
Figure 6: Garrondo. (2008, July 30). Alzheimer’s disease brain comparison. Wikimedia Commons. https://commons.wikimedia.org/wiki/File:Alzheimer%27s_disease_brain_comparison.jpg
